

- 2020-12-07 09:18-
Zirconia is an inorganic non-metallic material with high temperature resistance, corrosion resistance, abrasion resistance and excellent electrical conductivity, especially its excellent room temperature mechanical properties, high temperature resistance and corrosion resistance, and it is favored by scientific researchers. In the early 1920s It is applied to the field of refractory materials. Since the mid-1970s, outstanding countries in Europe, America and Japan have competed to invest in research and development of zirconia production technology and the production of zirconia series products, in-depth expansion of the application field of zirconia to structural materials and functional materials. At the same time, zirconia is also one of the key incentives for the development of high-performance new materials in the national industrial policy.

1. ZrO2 crystal structure
At high temperatures, ZrO2 belongs to a cubic fluorite structure. As shown in Figure 1, because the diameter of Zr4+ is larger than the diameter of O2- ions, it can be considered that Zr4+ forms a face-centered cubic lattice, occupying 1/2 of the octahedral gap, and O2- ions Occupies all four tetrahedral voids in the face-centered cubic lattice.
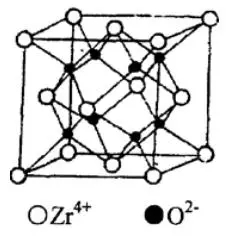
Figure 1
There are three crystal types of pure zirconia under normal pressure, monoclinic system at low temperature, density 5.68g/cm3, tetragonal system at high temperature, density 6.10g/cm3, and cubic system at higher temperature, density 6.27g/cm3 , The transformation relationship between the three crystal forms is as follows:
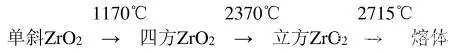
At room temperature, ZrO2 can only be a monoclinic phase. When it is calcined with zirconium salt to reach 650℃, a stable tetragonal phase appears. When it continues to rise, the tetragonal phase gradually transforms into a monoclinic phase. When the temperature continues to rise to 830℃, ZrO2 changes again. It begins to transform into tetragonal phase, and when it reaches 1170℃, it completely transforms into tetragonal phase, and when the temperature rises to 2370℃, it transforms into cubic phase; when the temperature decreases, it gradually transforms into tetragonal phase, and when it reaches room temperature, it becomes stable monoclinic phase. The transformation of monoclinic zirconia at 830 to 1200°C is more complicated and will cause hysteresis. It is this hysteresis phenomenon that provides a key performance for the application of zirconium dioxide in ceramics and refractory materials. During the transformation process, the corresponding volume change will occur. When the temperature rises, the volume shrinks by 5% when the temperature changes from the monoclinic phase to the tetragonal phase, and the volume expands when the temperature decreases from the tetragonal phase to the monoclinic phase. %, there are three phase structures, and their thermal expansion is different. Table 1 shows the lattice constants and densities of the three crystal forms of zirconia.
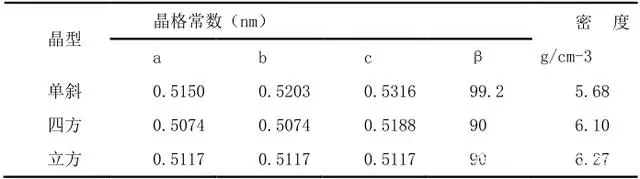
Table 1 The lattice constant and density of pure zirconia
2. The properties of zirconia and its role in refractory materials
In the oxide product system, ZrO2 contains many excellent characteristics, such as high melting point (2700℃), high structural strength at high temperature (200Kpa at 2000℃, can hold for 0.5~1 hour to produce deformation), good chemical stability, regardless of acid and Alkali or glass are all highly chemically inert, not easy to be wetted by liquid metal, so they also contain high metal stability (for many molten metals and even very active metals of group IV, V, VI contain good corrosion resistance), high temperature The vapor pressure and decomposition pressure are both low, and it contains lower volatility than Al2O3 and MgO.
ZrO2 has higher stability to vacuum than Al2O3 and MgO, which can be explained by the high bonding strength of zirconium and oxygen. The breaking energy of the Zr-O bond in ZrO2 is 757.8kJ/mol, but the Mg-O bond is 481.5kL/mol, and the Al-O bond is 418.7kJ/mol. The affinity of zirconium for oxygen and the strong Zr-O bond explain its higher metal stability than magnesia and alumina and lower interaction with carbon steel and decarburized steel. Therefore, it can be considered that ZrO2 can meet the technical requirements for smelting many pure metals and alloys at high temperature and high vacuum, and is a key refractory material for metallurgy in the future. Table 2 shows the key properties of refractories containing ZrO2.
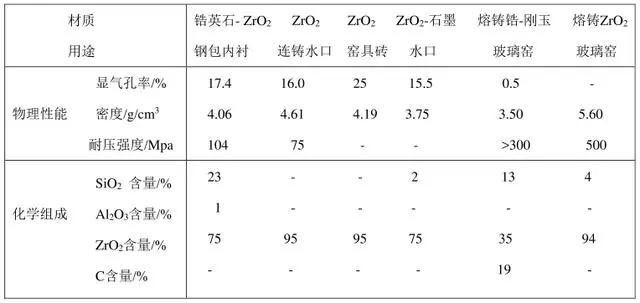
Table 2 Key properties of refractories containing ZrO2
Previous:Previous:What Effect Does the Grinding Medium Have on the Crushing Effect? Next: Next:What is the Difference Between Oxide Ceramics and Nitride Ceramics?